SV95C IS THE FIRST REGULATORY-QUALIFIED
REAL-WORLD DIGITAL OUTCOME MEASURE
FOR USE AS PRIMARY ENDPOINT QUALIFIED BY THE
EUROPEAN MEDICINES AGENCY (EMA)
Visit our official SYSNAV Healthcare website for more information!
SYSNAV Healthcare aims to unlock the potential of real-world data in the medical field by adapting precise motion-capture solutions to the needs of healthcare professionals and patients.
A trusted partner for your clinical programs
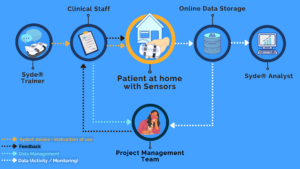
Sysnav Healthcare provides an all-in-one solution for drug developers to assess the efficacy of treatments. Our flagship product, Syde®, specializes in capturing accurate real-world movement data for patients with neuromuscular and neurological disorders.
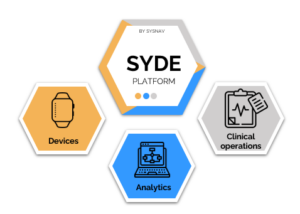
Our clinical-trial-ready solution encompasses:
- High-Precision Hardware and IT Infrastructure: We offer Syde, a patented high-precision wearable Digital Health Technology intricately integrated with advanced IT infrastructure.
- Advanced Analytics Services: Our services encompass both disease-specific and disease-agnostic variables. We are proud to have developed the first digital endpoint ever qualified by the European Medicines Agency (EMA) for Duchenne Muscular Dystrophy.
- Comprehensive Clinical Operation Services: Our comprehensive support includes guidance on Syde-related aspects of protocol writing, IRB clearance, logistics , wear compliance, site support, and effective data management.
We are committed to ensuring a seamless and effective clinical trial process, from beginning to end.
In 2023, along with the Duchenne Muscular Dystrophy (DMD) Community, we achieved a significant milestone as the European Medicines Agency (EMA) qualified SV95C (Stride Velocity 95th Centile) as a primary endpoint for superiority studies. This validation offers a valuable alternative to the 6 Minute Walk Test (6MWT) for evaluating ambulant DMD patients.
To learn more about our projects, Visit the SYSNAV Healthcare website.